| Numéro |
J. Phys. IV France
Volume 114, April 2004
|
|
|---|---|---|
| Page(s) | 431 - 437 | |
| DOI | https://doi.org/10.1051/jp4:2004114102 | |
J. Phys. IV France 114 (2004) 431
DOI: 10.1051/jp4:2004114102
Tetrathiapentalene-type donors containing
(thio)pyran-4-ylidene as a promising
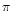 -electron framework
for multi-dimensional molecular conductors
-electron framework
for multi-dimensional molecular conductors
Y. Misaki1, T. Kaibuki1, K. Takahashi1, T. Nakayashiki1, K. Tanaka1, T. Kawamoto2 and T. Mori2
1 Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan
2 Department of Organic Materials, Tokyo Institute of Technology, O-okayama, Tokyo 152-8552, Japan
Abstract
The conducting materials based on
2-(1,3-dithiol-2-ylidene)-5-(pyran-4-ylidene)-1,3,4,6-tetrathiapentalene
(PDT-TTP) and its thiopyran and/or selenium analogues have been developed.
The ethylenedithio derivative (ET-PDT) affords 4:1 salt with PF
6-
anion (ET-PDT)
4PF
6(cn) (cn = 1-chloronaphthalene). It has the
so-called
![]() -type array of the donors with quasi one-dimensional
electronic structure due to strongly tetramerized stack. On the other hand,
(TM-TPDS)
2AsF
6 adopt the "windmill type" array of the donors. In
this salt, relatively large side-to-edge interactions stabilize their
electronic structure, while effective side-by-side interaction is inhibited
by methythio groups projected along the molecular short axis. In contrast,
edge-to-edge interactions are dominant in (SM-PDT)PF
6(PhCl)
x
through selenium-selenium contacts along the molecular long axis.
(ET-PDT)
4PF
6(cn) shows metallic conductivity down to 1.5 K, while
(TM-TPDS)
2AsF
6 is an organic metal exhibiting MI transition at 100
K. On the other hand, (SM-PDT)PF
6(PhCl)
x is a semiconductor with
relatively high conduuctivity of
-type array of the donors with quasi one-dimensional
electronic structure due to strongly tetramerized stack. On the other hand,
(TM-TPDS)
2AsF
6 adopt the "windmill type" array of the donors. In
this salt, relatively large side-to-edge interactions stabilize their
electronic structure, while effective side-by-side interaction is inhibited
by methythio groups projected along the molecular short axis. In contrast,
edge-to-edge interactions are dominant in (SM-PDT)PF
6(PhCl)
x
through selenium-selenium contacts along the molecular long axis.
(ET-PDT)
4PF
6(cn) shows metallic conductivity down to 1.5 K, while
(TM-TPDS)
2AsF
6 is an organic metal exhibiting MI transition at 100
K. On the other hand, (SM-PDT)PF
6(PhCl)
x is a semiconductor with
relatively high conduuctivity of
![]() S cm
-1 in spite
of 1:1 composition.
Key words. tetrathiapentalene - X-ray structure analysis -
electrical conductivity.
S cm
-1 in spite
of 1:1 composition.
Key words. tetrathiapentalene - X-ray structure analysis -
electrical conductivity.
© EDP Sciences 2004



